
Mission Statement
The Yang group focuses on developing multimodal operando electron microscopy and synchrotron-based X-ray methods to achieve an atomic/molecular-level understanding of electrochemical dynamics of energy materials at solid-liquid interfaces across multiple spatiotemporal scales
Research Overview
Electrochemistry lies at the interface of chemistry and energy materials and represents one of the most promising approaches for enhancing energy efficiency, mitigating environmental impacts and carbon emissions, and enabling renewable energy technologies. Our research focuses on the fundamental understanding of electrochemical mechanisms at interfaces with an emphasis on CO2 reduction, clean H2 production and rechargeable batteries. The central scheme of the Yang group lies in developing operando methods, based on advanced electron microscopy at Cornell Center for Materials Research (CCMR) and synchrotron based X-ray methods at Cornell High Energy Synchrotron Source (CHESS), to investigate fundamental aspects of solid-liquid interfaces related to renewable energy technologies.
Advance Carbon-Neutral and Hydrogen Based Energy Technologies
One of the grand challenges of our time is how to meet the increasing global energy needs in a sustainable and environmentally responsible way. While the use of fossil fuels has greatly improved our standard of living, it has also caused detrimental environmental consequences associated with their extraction and combustion. Shifting the energy landscape from fossil fuels to clean energy will play a key role in tackling the pressing carbon emission and climate change issues within the decade. Hydrogen has one of the highest energy densities (120 MJ/kg) of any fuel, making it a clean and efficient energy carrier to replace fossil fuels to store and distribute energy. Clean H2 can be used to power hydrogen fuel cell vehicles, which can dramatically lower the use of fossil fuels in transportation. At a larger scale, clean H2 has the potential to revolutionize chemical industries, such as power generation, ammonia production, oil refining and semiconductor processing, which produce annually more than 20 gigatons of CO2 and represent more than half of the global carbon emissions. Electrochemists are well positioned to develop hydrogen energy with water electrolysis that enables clean H2 production at scale with potentially zero carbon emissions if driven by renewable solar/wind energy. Another more radical approach is the direct electrochemical reduction of CO2 to valuable chemicals and liquid fuels, which would, in turn, enable closing the carbon cycle. Products of CO2 reduction, such as CO/H2 and C2H4, can serve as synthetic feedstocks for a variety of organic molecules and polymers when combined with clean H2. We focuses on the scientific understanding of fundamental factors governing electrocatalysis in H2 production and CO2 reduction for affordable alkaline electrolysis technologies at scale. In particular, we seek to understand molecular mechanisms of multiple proton-coupled electron transfer (PCET) processes in proton-deficient alkaline media spanning from hydrogen/oxygen evolution reaction (HER/OER) to significantly more challenging CO2 reduction reaction (CO2RR).
Multimodal Operando Methods at Solid-Liquid Interfaces
As electrochemists, we have the dream to directly resolve the complex nature of (electro)catalyst active sites and capture real-time “movies” of reaction dynamics, i.e. “watching chemistry in action”. The need for such fundamental understanding has stimulated the development of time-resolved operando/in situ methods at the nm-to-atomic scale, which have greatly enhanced our ability to identify activity descriptors of electrocatalysts and establish structure-property relationships of energy materials. As shown in the fishing analogy, ex situ (Latin, off site)methods provide a baseline understanding of pristine or postmortem samples while in situ (Latin, on site) methods simulate one or some of the reaction conditions but still deviate from realistic (device-level) operating conditions. Operando (Latin, operating) methods emphasize achieving multiple experimental conditions to fully sustain a working catalyst in an operating device.
In the Yang group, we push and define the frontiers of operando electrochemical liquid-cell scanning transmission electron microscopy (EC-STEM) and correlative synchrotron based X-ray methods, which are complementary tools to comprehensively investigate reaction dynamics across multiple spatiotemporal scales. Operando EC-STEM provides nm-to-atomic scale information of individual nanoparticles (NPs) in a localized environment, while synchrotron based X-rays interrogate a large ensemble of NPs with statistical analysis. In an effort to encourage greater adoption of advanced operando methods by the general electrochemistry community, we emphasize the importance to benchmark electrochemistry in those specialized liquid cells, which can faithfully represent measurements in a standard three-electrode electrochemical cell. We anticipate that multimodal operando methods will become indispensable for understanding interfacial reaction mechanisms for the broad chemistry and energy materials communities.


References:
1. Yang* et al. Curr. Opin. Electrochem. 2023, 42, 101403 (Link)
2. Yang et al. ACS Catal. 2021, 11, 1136 (Link)
Operando EC-STEM can directly visualize the dynamic evolution of electrocatalysts and provide valuable insights that were not previously accessible by conventional ex situ measurements. Operando EC-STEM enables quantitative electrochemistry and simultaneous quantitative STEM imaging, diffraction and spectroscopy at high spatiotemporal resolution. The recent development of the electron microscope pixel array detector (EMPAD) enables 4D-STEM diffraction imaging to retrieve nm-scale structural information at a much lower beam dose, which is indispensable for beam-sensitive materials in liquid. Our group demonstrates the first example of 4D-STEM in liquid, which provides unprecedented structural analysis beyond conventional STEM imaging of morphology. Operando electrochemical 4D-STEM in liquid, driven by machine learning, has shown great potentials to interrogate complex structures of active sites of energy materials at solid-liquid interfaces.


Operando EC-STEM reveal Cu nanograins for CO2 electroreduction
Quantitative electrochemistry on microelectrode chip


Operando EC-4D-STEM

Operando EC-STEM of Cu electrodeposition on Au nanocubes/Pt WE
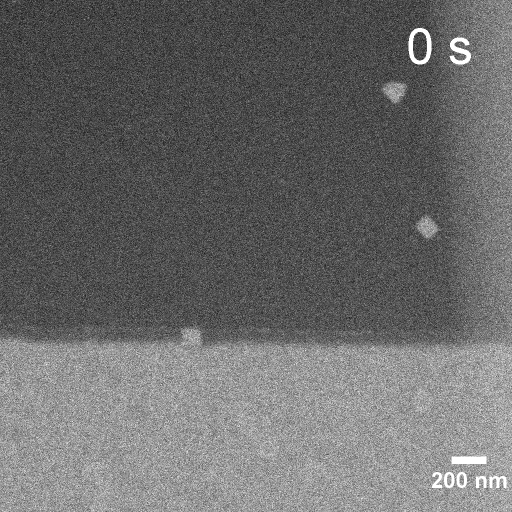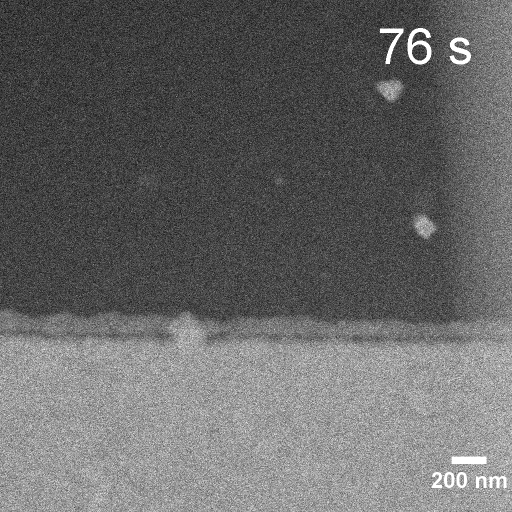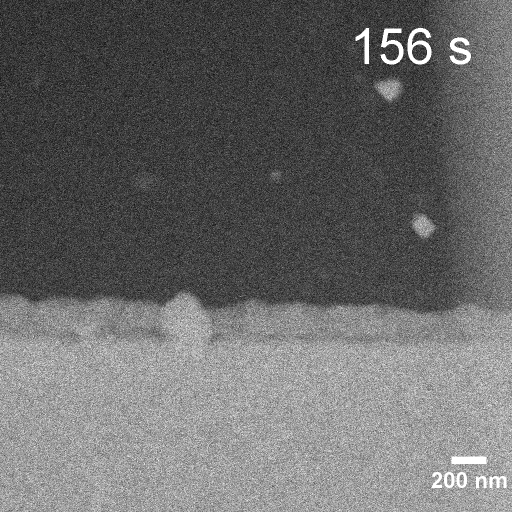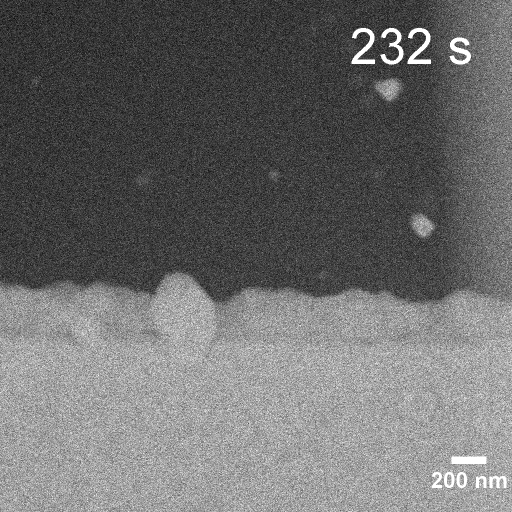
-0.1 V
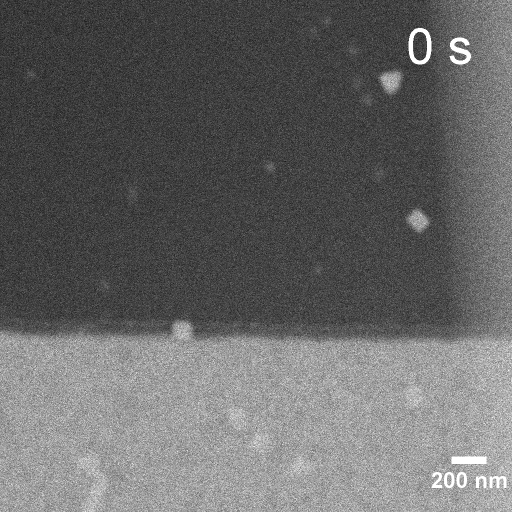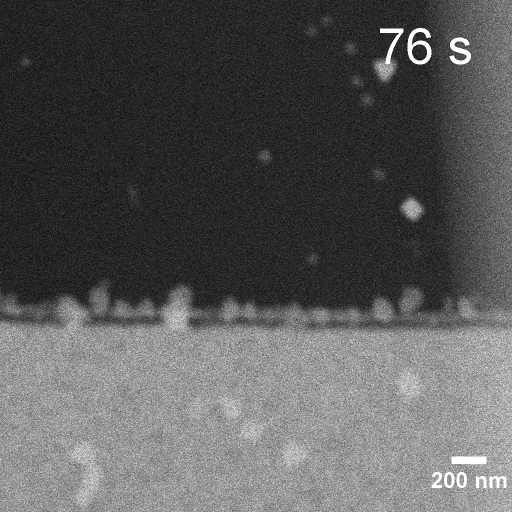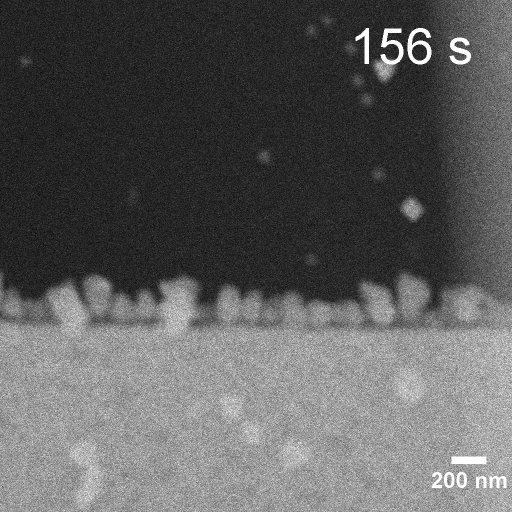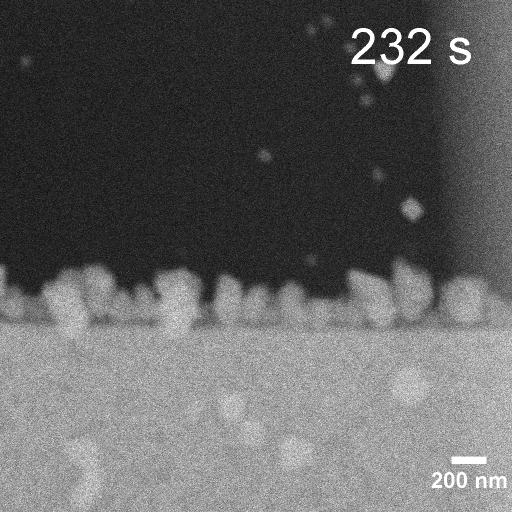
-0.2 V
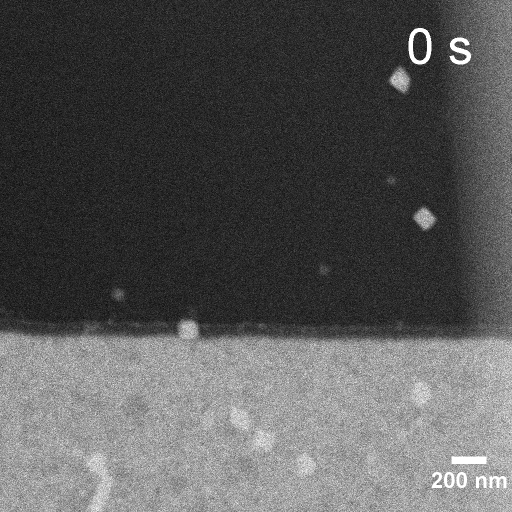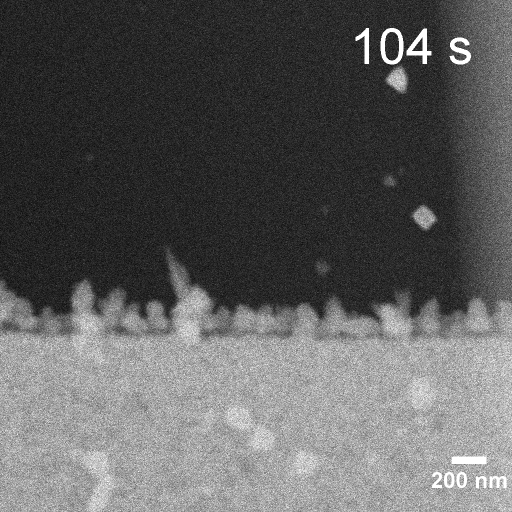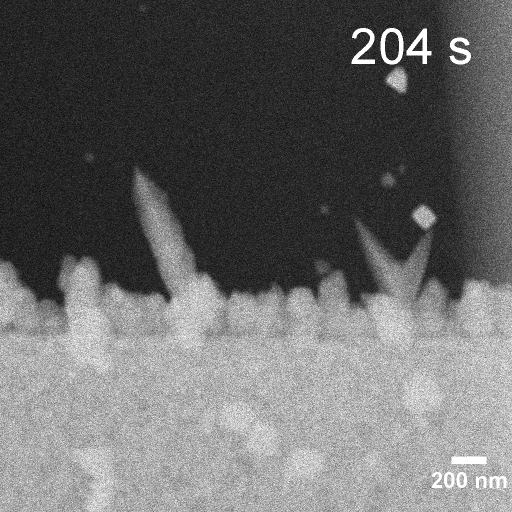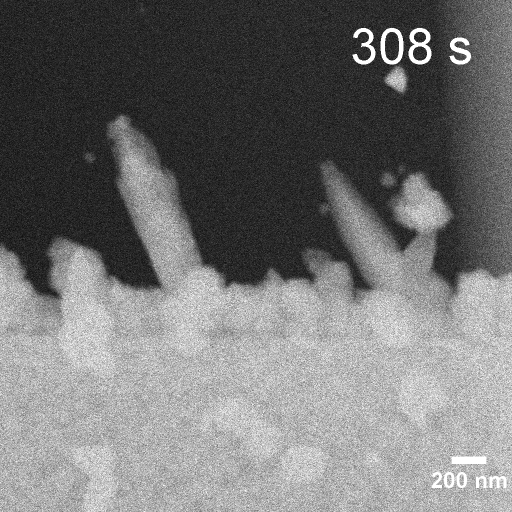
-0.3 V
References:
1.Yang et al. Nature 2023, 614, 262 (Link)
2.Yang et al. JACS 2022, 144, 15698 (Link)
3.Yang et al. ACS Energy Lett. 2022, 7, 1292 (Link)
Operando synchrotron based hard X-rays can penetrate penetrate mm or thicker samples in standard electrochemical cells or operating energy devices. Hard X-ray absorption near-edge structure (XANES)
and extended X-ray absorption fine structure (EXAFS), is the most widely used operando X-ray method for reliably probing changes in oxidation states and coordination environment during electrochemical reactions. XANES of first-row transition metals, collected in transmission mode, has an edge energy resolution of ~1.5 eV. Recently developed high resolution crystal spectrometer has given rise to high-energy-resolution fluorescence detected (HERFD) XAS, which enables much higher energy resolution on the order of 0.5–0.75 eV, by selecting one particular fluorescence decay channel and isolate the decay transition from a single orbital with a much longer lifetime, minimizing the energy broadening and sharpening the spectral features. In collaboration with Dr. Chris Pollock at CHESS, we develop operando HERFD XAS to extract unprecedented information from XANES pre-edges and show the desorption of monolayer ligand as Cu nanocatalysts experience dynamic surface reduction. Besides hard X-ray spectroscopy, we are also interested in developing operando surface-sensitive X-ray diffraction based techniques, including the crystal truncation rod (CTR) and X-ray standing waves (XSWs), enable an atomic-scale understanding of the single-crystal metal or oxide electrode-electrolyte interfaces by decoupling surface changes from the bulk substrate.


References:
Conventional XAS
1. Yang et al. JACS 2019, 141, 1463 (Link)
2. Xiong,† Yang† et al. JACS, 2019, 144, 8927 (Link)
3. Zeng† Yang† et al. Sci. Adv. 2022, 8, eabj1584 (Link)
HERFD XAS
3. Yang et al. Nature 2023, 614, 262 (Link)
4. Feijoo, Yang et al. JACS 2023, 145, 20208 (Link)
Operando synchrotron based soft X-rays are more advantageous as a probe for surface and thin film electronic structures due to their large absorption cross-section and chemical sensitivity. The main challenges facing soft X-ray studies are the beam-induced damage due to large inelastic scattering cross section and the design of vacuum-compatible liquid cells, which are similar to that in EC-STEM studies. In collaboration with Dr. Cheng Wang at advanced light sources (ALS), we develop the first operando electrochemical resonant soft X-ray scattering (EC-RSoXS) to enable simultaneously soft X-ray absorption spectroscopy to probe chemical environment of nanocatalysts and X-ray scattering to interrogate interparticle dynamics.



Reference:
1. Yang et al. JACS 2022, 144, 8927 (Link)
Operando Electrochemistry at Solid-Liquid Interfaces
Single-crystal Electrode/Liquid Interfaces
Single-crystal metals and oxides provide an ideal materials platform with atomic-level control of surface structure/composition to reveal the structure-(re)activity relationships/correlations of electrocatalysts. This project takes advantage of the design and fabrication of well-defined single-crystal metal electrodes by the Clavilier method (Cornell is one of the few places in the world with such a capability.) and single-crystal oxide superlattices by molecular beam epitaxy (MBE) at the NSF supported PARADIM (Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials) Facility. Single-crystal metals and oxides will serve as well-defined electrode surfaces, which can provide an accurate description of the structure of surface adsorbates for theoretical simulations and guide the design of efficient high performing electrocatalysts for H2 production. Insights gained here will help advance our understanding of interfacial electrocatalysis, water structure, electron transfer as well as ionic and potential gradients at charged interfaces, and eventually approach one of the fundamental challenges in physical chemistry, experimentally resolving the electrochemical double layer (EDL).

References:
1. Yang et al. Nat. Chem. 2023, 15, 271 (Link)
2. Briega-Martos, Herrero, Feliu, Electrochim. Acta 2017, 241, 497 (Link)
Well-defined Nanocatalyst/Liquid Interfaces
Shape-controlled nanocrystals (1-100 nm) exclusively/selectively expose certain facets that can enable tunable catalyst activity/selectivity. They can bridge the knowledge gap between bulk single-crystal electrodes in the electrochemistry community and practical nanoparticles in the materials science community. While there are numerous studies on the synthesis of shape-controlled nanocrystals and characterization of their pristine structures, few studies have attempted to investigate how they activate and/or evolve/deactivate under electrochemical conditions. The project focuses on the design and colloidal synthesis of size- and shape-controlled 0D and 1D metal and oxide nanocrystals and their assembly into 2D and 3D periodic architectures as model systems for operando mechanistic studies. Fundamental insights gained from these studies will enable the rational design of high-performance CO2 reduction electrocatalysts with tunable selectivity and activity.

Practical Energy Materials Interfaces
The methodologies, developed based on bulk single crystals and shape-controlled nanocrystals, can be applied to tackle complex and realistic energy material interfaces. For instance, the polymer/ionomer/catalyst interfaces have been widely proposed but poorly understood due to the lack of necessary nm-scale time-resolve operando methods to probe gas-solid-liquid triphase interfaces. We plan to develop operando X-ray methods that are compatible with realistic operating water and CO2 electrolyzers. Another important example is the formation of the solid-electrolyte interphase (SEI) in lithium batteries, which determines the cycle life, charge rate and safety of batteries.
Water/CO2 Electrolyzers

Lithium Batteries







